Pulmonary Medicine blog
Its Tuesday and here is the answer for the chest Medicine challenge 2.
Raise your hand if you answered D to Friday's CHEST Challenge question! Nice work! Read on for complete rationale.
Raise your hand if you answered D to Friday's CHEST Challenge question! Nice work! Read on for complete rationale.
A 45-year-old nonsmoking woman was referred for an opinion
regarding management of recurrent pneumothorax. She was well until age 27 when
she had a right-sided spontaneous pneumothorax. Two years later, she had
another right-sided pneumothorax and underwent thoracotomy and stapling of the
right lung apex. She has had no further episodes since that time. She is seeing
a dermatologist for multiple facial papules, but otherwise, her general health
is excellent. She denies any respiratory symptoms. Results of a physical
examination are normal other than multiple skin-colored papules over the
central face and nose. Results of a chest radiograph demonstrated right apical
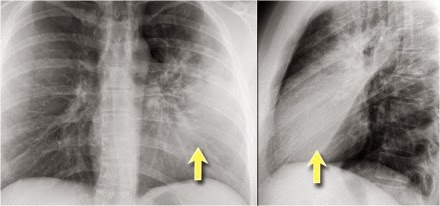
pleural thickening but were otherwise normal. A chest CT was performed and
representative images are shown in Figures 138-A and 138-B. Her lung function
was normal. The most likely diagnosis of this condition is:
A. Langerhans cell histiocytosis.
B. Lymphangioleiomyomatosis (LAM).
C. Sarcoidosis.
D. Birt-Hogg-Dubé syndrome (BHDS).
B. Lymphangioleiomyomatosis (LAM).
C. Sarcoidosis.
D. Birt-Hogg-Dubé syndrome (BHDS).
The CT scan reveals several cysts in the left lung and scarring
at the right apex related to the previous surgery. A history of recurrent
pneumothorax, lung cysts, and skin lesions (fibrofolliculomas) with normal lung
function is consistent with the diagnosis of Birt-Hogg-Dubé syndrome (BHDS)
(choice D is correct). Langerhans cell histiocytosis is characterized by
diffuse cystic disease of the lung, spontaneous pneumothorax, and airway
obstruction related to cigarette smoking. These findings are not present in
this patient (choice A is incorrect). Similarly, airflow obstruction, recurrent
pleural effusions, and diff use pulmonary disease are characteristic of LAM,
features that also are not found in this patient (choice B is incorrect).
Sarcoidosis is a granulomatous inflammatory lung disease characterized by diff
use parenchymal opacities, airflow obstruction, and possibly, skin lesions.
Lupus pernio, one of the skin manifestations of sarcoidosis, appears as purple
nodules on the nose, cheeks, and ears, and none of these features is present in
this patient (choice C is incorrect).
BHDS is an autosomal dominantly inherited genodermatosis that
predisposes a person to the development of cutaneous hamartomas
(fibrofolliculomas), kidney neoplasms, lung cysts, and spontaneous
pneumothorax. The BHD locus has been mapped to the short arm of chromosome
17(17p11.2). BHD is composed of 14 exons, and more than 40 unique mutations in
BHD have been reported. Most BHD germline mutations are frameshift or nonsense
mutations that are predicted to truncate the BHD protein, folliculin. Patients
exhibit multiple 1- to 5-mm white-colored or skin-coloured papules distributed
over the face, neck, and/or upper trunk, which histologically, are
fibrofolliculomas (Fig 138-C). BHDS is associated with a unique histologic
spectrum of bilateral and multifocal kidney tumors ranging from hybrid
oncocytic (67%) to chromophobe renal carcinoma (23%) to oncocytic renal
carcinoma (3%). Clear cell renal cell carcinoma (3%) has also been reported in
a few patients with BHDS. Pulmonary manifestations are a major feature of BHDS.
Most patients who are affected (89%) have multiple pulmonary cysts. The number
of lung cysts is clearly related to the risk of pneumothorax. Approximately
one-quarter of patients with BHDS have a history of one or more pneumothoraces.
Smoking does not appear to be a risk factor for pneumothorax in this
population.
Toro JR, Wei1 MH, Glenn GM, et al. BHD mutations, clinical and
molecular genetic investigations of Birt-Hogg-Dubé syndrome: a new series of 50
families and a review of published reports. J Med Genet. 2008;45(6):321-331.
Toro JR, Pautler SE, Stewart L, et al. Lung cysts, spontaneous
pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé
syndrome. Am J Respir Crit Care Med. 2007;175(10):1044-1053.
Pavlovich CP, Grubb RL III, Hurley K, et al.
Evaluation and management of renal tumors in the Birt-Hogg-Dubé syndrome. J
Urol. 2005; 173:1482-1486.